Key takeaways for Batteries
Batteries may seem like simple power sources but they are actually small electrochemical wonders that rely on a delicate “redox” reaction to move ions through an electrolyte while pushing life-giving electrons through your devices. When choosing the right fit it is all about matching the personality of the battery to your needs. Single-use Primary cells are your reliable long-term companions for low-drain gadgets while rechargeable Secondary batteries are the high-performance workhorses for everything you use daily. To get the best results, prioritize Energy Density if you need long runtimes, or Power Density if your device needs a sudden burst of energy. Just keep in mind that under heavy loads, especially with Lead-Acid types, Peukert’s Law tells us you might get less usable capacity than expected. For a long and healthy battery life especially with Li-ion try to stay in the “sweet spot” of 20–80% charge and keep things cool. Whether you’re opting for the portability of Li-ion or the rugged safety of LiFePO4, always ensure your setup includes a Battery Management System (BMS) and follows standards like IEC 62133 to keep your projects safe and professional.
Introduction to Batteries and Their Working Principles
Ever picked up a flashlight or your phone and thought about what keeps it running?. That little power pack we call a battery is doing some serious work behind the scenes. Batteries are everywhere your car your laptop even your smoke detector and they are all different built for specific jobs. Lets take a walk through the world of batteries exploring what they are, how they work and why they matter in our daily lives. This is not just about tech specs its about understanding the magic that powers our world. Global battery demand is expected to grow more than five-fold by 2030, with lithium-ion technologies projected to represent over 90% of that market. Understanding battery chemistry isn’t just theoretical it’s essential for matching performance, cost, and safety.
What’s Inside a Battery?
Picture a battery as a tiny chemical factory. Its got a couple of key players an anode (the negative side) a cathode (the positive side) and an electrolyte that lets ions shuffle between them. When you connect a battery to a device a chemical reaction kicks off electrons flow from the anode to the cathode through your device creating the electricity that makes things go. Its like a dance where the anode gives up electrons, the cathode grabs them and the electrolyte keeps everything in balance. This basic setup called an electrochemical cell, is the heart of every battery whether its powering a toy or a Tesla. For a closer look at how these components come together, check out Wikipedia: Electrochemical Cell. 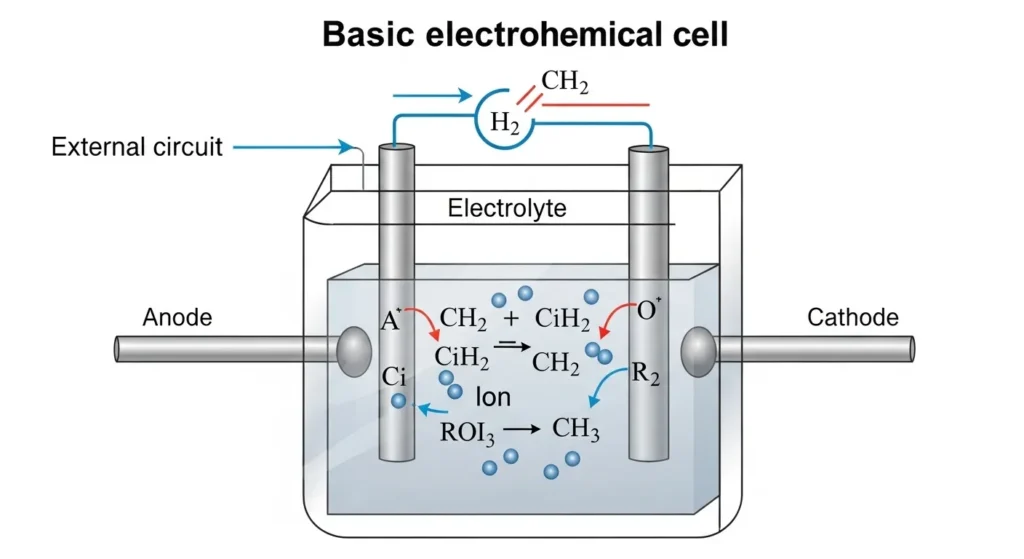
Quantitative Principles: Energy, Power, and Peukert’s Law
A battery’s performance is not static; it is governed by measurable quantitative principles that define its capacity, energy density, and discharge rate.
1. Energy vs. Power Density
Professionals differentiate between energy and power density. Energy Density Wh/kg defines how long a battery will last (capacity), while Power Density W/kg defines how much current (Amps) the battery can deliver in a burst (acceleration or high-drain tools).
- Energy Density (Wh/kg): Energy Density=Battery Voltage⋅Capacity(Ah)Weight(kg)
- Power Density (W/kg): Measured as the maximum power output divided by the battery’s weight. Li-ion typically has high energy density, while LiPo excels at high power density.
2. Peukert’s Law: The Capacity Killer
For Lead-Acid and other non-Lithium chemistries, a battery’s usable capacity decreases significantly as the discharge current (I) increases. This relationship is defined by Peukert’s Law:
Where C_p is the theoretical capacity, I is the current, t is the discharge time, and k is the Peukert constant (a factor representing internal resistance, always greater than 1). A battery rated for 100 Ah over 20 hours may only deliver 60 Ah if discharged over 1 hour.
The Two Big Families: Primary and Secondary Batteries
Batteries come in two main flavors primary and secondary. Primary batteries are the one-and-done type you use them until they are drained then toss them. Think of the AA batteries in your TV remote they are simple cheap and great for devices that don’t need a lot of juice over time. Secondary batteries, on the other hand are rechargeable you can zap them back to life with a charger. Making them perfect for things like your phone or electric car where you’re constantly using and reusing power. This distinction matters because it shapes what a battery can do and how you use it. Primary batteries are like a quick snack convenient but gone once you’re done. Secondary batteries are more like a reusable water bottle, ready to be refilled for the long haul. To dig deeper into battery classifications, see BBC Science: How Batteries Work.
Primary Batteries: The Single-Use Heroes
Lets start with primary batteries. These are the ones you probably grew up with, popping them into a Game Boy or a wall clock. A common example is the zinc-carbon battery, which gives you about 1.5 volts. Its got a zinc anode and a manganese dioxide cathode with an electrolyte like ammonium chloride keeping things moving. These are affordable and great for low-drain stuff like remote controls or flashlights. Then there the alkaline battery also 1.5 volts but with more staying power thanks to its zinc and manganese dioxide combo in a potassium hydroxide electrolyte. You will find these in everything from toys to smoke alarms. There also the lithium battery not to be confused with lithium-ion which pumps out around 3 volts and lasts a long time. These are the go-to for things like cameras or medical devices, where you need reliable power without frequent replacements. A user on an electronics forum once shared, I swapped out my smoke detector’s alkaline batteries for lithium ones, and they have lasted three years without a peep. That kind of longevity is why lithium batteries are a favorite for critical devices. 
Secondary Batteries: The Rechargeable Workhorses
Now, lets talk about secondary batteries the ones you can recharge. These are the backbone of our tech-heavy world. Take lead-acid batteries for example. They are the heavy hitters in your car delivering 2 volts per cell with lead plates and a sulfuric acid electrolyte. They are rugged reliable and great for starting engines or powering UPS systems. But they are heavy and need occasional maintenance like topping up the electrolyte. Then there the nickel-cadmium (NiCd) battery which gives about 1.2 volts per cell. These are tough as nails, used in power tools and older cordless phones but they have got a downside called the memory effect if you don’t fully discharge them before recharging they can lose capacity. A hobbyist I read about on a DIY blog said “My NiCd drill battery still works after a decade but I have to be careful not to charge it halfway”. Lithium-ion batteries are the rock stars of the rechargeable world powering your phone laptop and electric vehicles. With 3.7 volts per cell and a lightweight design, they pack a lot of energy. Their secret? A lithium compound cathode a graphite anode, and a liquid or gel electrolyte. They’re efficient but can be finicky overheating or overcharging can cause trouble, which is why your phone has all those safety circuits. For a detailed look at rechargeable batteries, visit HowStuffWorks: Battery Types.
How Batteries Actually Work
So, how does a battery turn chemicals into electricity? It’s all about a redox reaction short for reduction-oxidation. At the anode, a material like zinc in a zinc-carbon battery oxidizes, shedding electrons that flow through your device to the cathode, where something like manganese dioxide grabs them. Meanwhile, ions move through the electrolyte to keep the charges balanced. It’s a bit like a bucket brigade passing water: electrons move one way, ions another, and your device gets the power it needs. Take a lithium-ion battery. The lithium ions shuttle between the anode and cathode during charging and discharging, while electrons flow through the circuit to do the work. It’s a delicate balance, and if you’ve ever noticed your phone getting warm while charging, that’s the battery working hard to manage that flow. 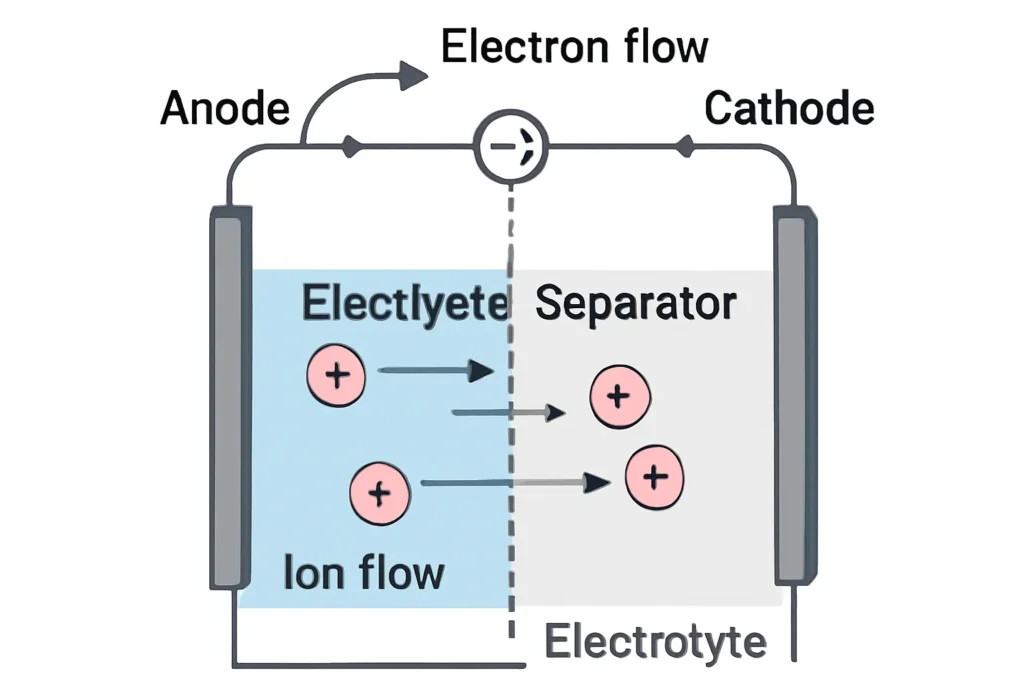
Comparing Battery Types: Which One Right for You ?
Choosing the right battery depends on what you need it for. Here’s a quick comparison to help you decide:
| Battery Type | Voltage | Rechargeable? | Common Uses | Pros | Cons |
| Zinc-Carbon | 1.5V | No | Remote controls, flashlights | Cheap, widely available | Short lifespan, low power output |
| Alkaline | 1.5V | No | Clocks, toys, smoke alarms | Long shelf life, reliable | Not rechargeable, disposal issues |
| Lithium | 3V | No | Cameras, medical devices | Long lifespan, high voltage | Expensive, not rechargeable |
| Lead-Acid | 2V/cell | Yes | Cars, UPS systems | Durable, high power output | Heavy, requires maintenance |
| Nickel-Cadmium (NiCd) | 1.2V/cell | Yes | Power tools, cordless phones | Robust, good for high-drain use | Memory effect, contains cadmium |
| Lithium-Ion | 3.7V/cell | Yes | Phones, laptops, EVs | High energy density, lightweight | Can overheat, expensive |
This table should give you a sense of what each battery brings to the table and where it shines or doesn’t. For a more focused comparison of popular rechargeable options, here’s a chart on Li-ion vs. NiMH vs. Lead-Acid vs. LiFePO4 (a safer variant of Li-ion):
| Battery Type | Energy Density (Wh/kg) | Cycle Life | Weight | Cost | Safety | Best For |
| Lithium-Ion (Li-ion) | 150-250 | 500-1,000 | Light | Medium-High | Moderate (risk of thermal runaway) | Portable electronics, EVs |
| Nickel-Metal Hydride (NiMH) | 60-120 | 300-500 | Medium | Low-Medium | High | Power tools, hybrid cars |
| Lead-Acid | 30-50 | 200-300 | Heavy | Low | Moderate (acid leaks possible) | UPS, car starters |
| Lithium Iron Phosphate (LiFePO4) | 90-160 | 2,000+ | Medium | Medium | Very High (stable, no fire risk) | Solar storage, e-bikes |
You can see the video for “changing a car key battery”
Where Batteries Show Up in Your Life
Batteries are everywhere, quietly making your day easier. That alkaline battery in your kid’s toy keeps the noise going (for better or worse). The lithium-ion battery in your phone lets you scroll through social media or navigate a new city. Lead-acid batteries get your car started on a cold morning, and lithium batteries in medical devices like pacemakers keep people safe. Even in big setups like data centers or hospitals batteries like sealed maintenance-free (SMF) ones ensure the lights stay on during a power outage. I remember when my car battery died in the middle of nowhere. A quick jump-start got me going, but it made me appreciate how much we rely on these little powerhouses. Whether it’s a tiny button cell in a watch or a massive battery bank in an electric vehicle, batteries are the unsung heroes of our connected world. For more on battery applications, read Scientific American: The Future of Batteries.
Real-World Examples:
Drones and High-Discharge Robotics
For applications demanding intense bursts of power and minimal weight, such as drones (UAVs) and high-performance robotics, Lithium Polymer (LiPo) batteries are the undisputed standard. LiPo chemistry offers the highest power-to-weight ratio, which is critical for achieving agile flight and rapid acceleration. A typical racing drone requires a 4-cell (4S) pack operating at 14.8 V with a capacity in the 1300 mAh to 2200 mAh range, specifically chosen for its high C-rate (discharge current capability). For projects prioritizing extended flight time or endurance over speed, Lithium-Ion (Li-ion) cells, often in the 18650 format, are preferred. While Li-ion offers superior energy density (more Wh/kg for longer runtime, often 20+ minutes), it is heavier and has a lower instantaneous power output than LiPo. Designers must always utilize a robust Battery Management System (BMS) for LiPo to mitigate the risk of thermal runaway associated with its high discharge potential.
Uninterruptible Power Supply (UPS) Systems
UPS systems require highly reliable batteries capable of delivering high surge current immediately upon grid failure. Valve-Regulated Lead-Acid (VRLA) batteries, specifically their absorbed glass mat (AGM) variant, remain the cost-effective industry standard due to their proven reliability and deep discharge tolerance over short durations. A common configuration for a small home UPS is a 12 V, 7 Ah VRLA unit, expected to last 3-5 years with proper float charging. For enterprise UPS systems or those operating in high-temperature environments (where VRLA batteries degrade quickly), Lithium-Ion or Nickel-Cadmium (NiCad) chemistries are superior. NiCad offers exceptional robustness and tolerance to heat, while Li-ion provides a significantly longer cycle life and higher energy density in a much smaller footprint, justifying the higher initial cost.
Arduino and Portable Electronics Projects
For microcontroller platforms like Arduino and general portable electronic setups, the primary requirements are stable voltage regulation, good energy density, and reusability. Rechargeable Lithium-Ion cells (e.g., the versatile 3.7 V 18650 format) are the most suitable choice due to their high voltage per cell, requiring fewer cells for the desired output. They provide a stable 5 V to 9 V output when paired with a simple buck/boost regulator, lasting hours longer than disposable alternatives. For low-power or low-current projects, Nickel-Metal Hydride (NiMH) AA packs offer a safe, rechargeable option without the strict handling requirements of lithium chemistry. Crucially, 9 V Alkaline batteries should be avoided for long-term power solutions as their voltage drops off quickly under continuous load, severely limiting both capacity and runtime.
Keeping Your Batteries Happy
Taking care of your batteries requires focused attention on charge levels and temperature. For Lithium-Ion (Li-ion) batteries, maximizing lifespan means avoiding deep discharge and high charge states; ideally, maintain them between 20% and 80% charge. Always use a charger equipped with an auto-cutoff circuit to prevent damaging overcharging. Lead-Acid batteries require different maintenance: ensure the electrolyte levels are checked (if not a sealed unit) and periodically apply an equalization charge to prevent performance degradation from cell imbalance.
All battery types should be stored in a cool, dry place, as heat is the primary catalyst for accelerated chemical wear. This wear is tied directly to the electrochemical reaction: energy is generated through a redox reaction where the anode undergoes oxidation (releasing electrons) and the cathode undergoes reduction (accepting electrons). The electrolyte facilitates the movement of ions internally to maintain charge balance, completing the circuit and supplying power. , visit The Guardian: Battery Recycling Challenges.
- “Follow international safety standards such as IEC 62133 (for rechargeable sealed battery safety) and UL 2054 for household/commercial battery systems.”
- More precise storage temp: “Store batteries ideally between 5 °C – 25 °C (41-77 °F); avoid above 45 °C (113 °F) which degrades lifespan substantially.”

Deep Dive: Failure Modes and Troubleshooting
Understanding why batteries fail is key to maximizing their lifespan and ensuring safety in critical applications.
Lithium-Ion: Thermal Runaway and BMS
The primary safety concern for Li-ion is thermal runaway , a catastrophic process where excessive heat (caused by overcharging, internal shorts, or physical damage) leads to a rapid, self-sustaining temperature increase. This can cause fire or explosion.
The Fix: All Li-ion packs require a Battery Management System (BMS) . The BMS is a critical safety circuit that monitors and prevents cells from exceeding safe voltage/current limits and detects temperature spikes, ensuring compliance with safety standards.
Lead-Acid: Sulfation and Stratification
Lead-acid batteries typically fail due to two internal physical processes:
- Sulfation: A build-up of hard lead sulfate crystals on the plates when the battery is left discharged for too long. This decreases the effective surface area for chemical reaction. Fix: Apply a controlled overcharge called an equalization charge to dissolve the sulfate crystals.
- Stratification: The sulfuric acid electrolyte separates, with denser acid sinking to the bottom. The top is less conductive, which reduces capacity. Fix: Use specific chargers or periodically apply a controlled overcharge to “mix” the electrolyte through gassing.
Industry Standards and Regulatory Compliance
Any battery used in commercial, medical, or consumer products must meet rigorous international and national safety and performance standards.
1. Safety and Certification Standards
- IEC 62133: The global standard governing the safety requirements for portable sealed rechargeable cells (Li-ion, NiMH). This is essential for international manufacturing and sales.
- UL 2054: A widely recognized US standard for the safety testing of household and commercial batteries. Compliance ensures the product meets safety benchmarks for fire, chemical, and electrical hazards.
2. Performance and Testing Standards
The performance specifications listed on data sheets are often defined by IEC testing protocols:
- IEC 61960: Defines how to test and rate the capacity and cycle life of secondary lithium batteries. This ensures manufacturers’ capacity claims are standardized and comparable.
- Transportation (UN 38.3): Any lithium battery transported by air, sea, or road must pass the UN 38.3 testing regime, which simulates pressure, temperature extremes, vibration, and shock to prevent thermal events during shipping.
Looking Ahead: The Future of Batteries
Batteries are evolving fast. Researchers are working on solid-state batteries, which use a solid electrolyte for better safety and energy density imagine electric cars that go farther on a single charge. There’s also a push for greener materials to reduce the environmental impact of mining things like cobalt. Recycling is another big focus, with new methods to recover more from old batteries, cutting down on waste. I recently read about a startup developing batteries with plant-based materials, which sounds wild but could be a game-changer for sustainability. It’s exciting to think about where this tech is headed, making our devices more efficient and our planet a little happier. For the latest trends in battery technology, check out CNN: Innovations in Battery Technology.
- Solid-state batteries are expected to offer up to twice the energy density of current lithium-ion designs, with commercialization likely around 2027-2030 as major companies like Toyota, Samsung, and QuantumScape scale up R&D.”
- “Recycling technologies are improving: modern processes now recover over 90% of key metals (like cobalt, nickel), and circular economy models aim for reuse of battery modules in second-life applications.”
Battery Selection Flowchart (Text-Based)
Start → What is your main priority?
Need long backup time?
→ Choose Lead-Acid or LiFePO4
(Ideal for UPS systems, solar power setups)
Need something lightweight and portable?
→ Choose Li-ion or LiPo
(Great for drones, mobile devices, wearables)
Looking for low cost and simplicity?
→ Choose NiMH or Alkaline
(Suitable for toys, remotes, Arduino projects)
Want high safety and no maintenance?
→ Choose LiFePO4 or Sealed VRLA
(Safe, durable, and maintenance-free options)
Note on Battery Safety
Always handle batteries with care:
- Li-ion and LiPo batteries can overheat, swell, or catch fire if overcharged, short-circuited, or punctured.
- Always use proper chargers and follow manufacturer guidelines.
- Dispose of batteries responsibly — never throw them in regular trash.
| Battery Type | Chemistry | Typical Uses | Advantages | Limitations |
|---|---|---|---|---|
| Alkaline | Zinc-Manganese dioxide | Remote controls, flashlights | Long shelf life, inexpensive | Non-rechargeable |
| Lead-Acid | Lead dioxide & lead | Automotive starters, UPS systems | High surge current capacity | Heavy, toxic, limited cycle life |
| Lithium-ion (Li-ion) | Lithium cobalt oxide, lithium iron phosphate | Smartphones, EVs, laptops | High energy density, rechargeable | Sensitive to temperature |
| Nickel-Metal Hydride (NiMH) | Nickel oxide hydroxide & metal hydride | Power tools, cameras | Rechargeable, less toxic | Memory effect, moderate energy |
| Solid-State | Solid electrolytes | Emerging EV tech, wearables | Safer, higher energy potential | Currently expensive, developmental |
Frequently Asked Questions About Batteries
How do lithium-ion batteries work?
Lithium-ion batteries power devices through a redox reaction. Lithium ions move from the anode (usually graphite) to the cathode (a lithium compound) through an electrolyte, while electrons flow through the device to create electricity. Charging reverses this process.
What’s the difference between energy density and power density?
Energy density determines how long a device can run on a charge (runtime), while power density indicates how quickly it can deliver energy (burst current). Laptops favor energy density, whereas drones favor power density for rapid acceleration.
Why does my lead-acid battery seem to “shrink” under heavy load?
Peukert’s law explains that higher discharge currents reduce usable capacity. Drawing large currents shortens runtime compared to the rated capacity at low currents.
Is LiFePO4 safer than standard Li-ion?
LiFePO4 batteries are more thermally stable and less prone to thermal runaway. They have long cycle life and consistent performance, making them ideal for solar storage and e-mobility applications.
How should I store batteries to maximize lifespan?
Keep batteries cool and dry. Avoid heat, which accelerates chemical wear. Store Li-ion batteries at 40–60% charge if unused for long periods. Lead-acid batteries should maintain proper float charge to prevent sulfation.
Do I need a BMS for all lithium packs?
Yes. A Battery Management System (BMS) protects against over/under-voltage, over-current, and temperature excursions. It balances cells and reduces the risk of thermal events, especially in multi-cell packs.
How to change the battery of a car key?
Use a small tool to open the key fob, remove the old battery (usually CR2032), insert a new one with correct polarity, and close the fob. Check your car manual for model-specific instructions.
How to check battery health?
Battery health depends on the device:
- Android: Settings > Battery > Battery Health or apps like AccuBattery.
- iPhone/iPad: Settings > Battery > Battery Health & Charging. For iPads, third-party apps may be needed.
- Laptop: Windows: powercfg /batteryreport or Settings > System > Battery; Mac: System Settings > Battery; third-party apps like HWMonitor provide more details.
- Samsung: Settings > Device Care > Battery or dial *#0228#.
How do I calculate battery capacity?
Battery capacity (C) is calculated as C = I × t, where I is current in amperes and t is time in hours. For example, a 12 V, 100 Ah battery stores 1.2 kWh of energy.
Which battery is best for solar systems?
Lithium-ion batteries are ideal due to high energy density, long lifespan, and efficiency compared to lead-acid batteries.
What standards govern battery safety?
Battery safety is governed by standards such as IEC 61960 (lithium-ion performance), IEEE 1188 (lead-acid maintenance), and UL 2054 (household/commercial safety).