Wavelength Order Explained (Chart, Frequency & Exam Traps)
Quick Answer for Students: In the VIBGYOR spectrum, Red has the longest wavelength while Violet has the shortest wavelength and highest frequency. This order is critical for physics exams and numerical problems.If you are preparing for Class 9, Class 10, FSC, O-Levels, or competitive exams, this guide explains VIBGYOR in a way textbooks usually don’t.Why VIBGYOR Wavelength Questions Are Asked in Exams
Most students lose marks because they memorize VIBGYOR as colors only not as a wavelength and frequency scale. Examiners often twist questions like:- Which color bends the most in a prism?
- Which VIBGYOR color has maximum frequency?
- Arrange colors in increasing wavelength order
Introduction to Wavelength of Vibgyor
Imagine staring at a rainbow, its colors stretching across the sky in a perfect arc. That stunning display is all thanks to wavelength, the invisible force behind light, sound, and so much more. But have you ever wondered why the human eye only sees these specific seven colors, or why Red always takes the outer edge while Violet stays inside? Wavelength is like the heartbeat of waves, and understanding its precise measurements is the key to unlocking the science of the visible spectrum.
In this guide, we’re diving deep into the world of wavelength, with a special focus on the Wavelength and VIBGYOR in Physics spectrum. We won’t just give you the basic definitions; we’ll explore the exact nanometer ranges that separate each hue and why even a slight shift in frequency can change a color entirely. From the glow of your phone screen to the precision of ultraviolet lasers, we break down the complex physics into a simple, engaging format including a detailed color-to-wavelength chart that explains the “why” behind the VIBGYOR sequence. Whether you’re a student or a science enthusiast, this breakdown will show you what most textbooks leave out about optics and acoustics.
VIBGYOR Wavelength Order (Most Asked Question)
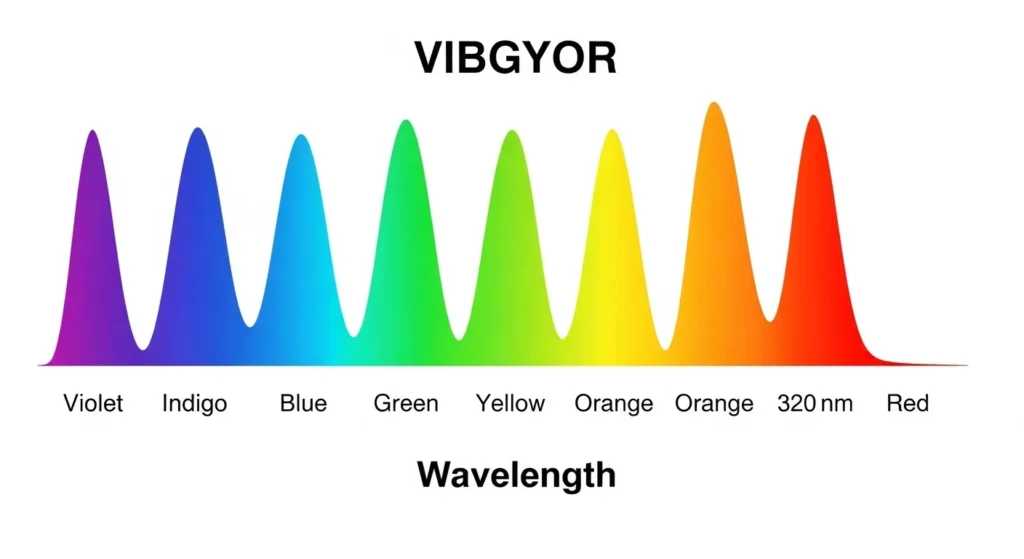
Correct Order from Shortest to Longest Wavelength:Violet → Indigo → Blue → Green → Yellow → Orange → Red Exam Tip:
Shorter wavelength = Higher frequency = Higher energy
Longer wavelength = Lower frequency = Lower energy
Exam Tip:
Shorter wavelength = Higher frequency = Higher energy
Longer wavelength = Lower frequency = Lower energyWhy Red Has the Longest Wavelength (Conceptual Explanation)
Red light bends the least when passing through a prism because its wavelength is the longest. A longer wavelength means a lower refractive index inside glass, which causes less deviation. This is why:- Red appears on the outer edge of a rainbow
- Red is used in stop signs and danger signals
- Red light travels further through fog and dust
Why Violet Has the Maximum Frequency
Violet light has the shortest wavelength, and according to the equation:c = λ × f
When wavelength (λ) decreases, frequency (f) must increase. That is why violet carries the highest photon energy in the visible spectrum.What is Wavelength? (The Physics Breakdown)
Wavelength is the distance between two identical points on a wave think of it as the distance from the crest (peak) of one ripple to the crest of the next.
Courtesy of ShutterstockKey Concepts at a Glance:
Symbol: Represented by the Greek letter λ (lambda).
Measurement: Usually measured in meters (m), nanometers (nm), or angstroms (Å).
The Golden Rule: In physics, wavelength and frequency are tied together by this fundamental equation:
c=λf(Where c is the speed of light, approximately 300 million meters per second).
How Wavelength Shapes Our World
Wavelength isn’t just a number; it determines how we perceive energy:
Visible Light: It paints the sky with colors (VIBGYOR). For example, the Violet wavelength is short and high-energy, while the Red wavelength is long and carries less energy.
Acoustics: It carries the music to your ears as audio waves.
Radiation: From the warmth of Infrared on your skin to the high-energy precision of Ultraviolet lasers.
| Wave Type | Wavelength Characteristic | Energy Level |
| Short Wavelength (e.g., Violet/UV) | High Frequency | High Energy |
| Long Wavelength (e.g., Red/Infrared) | Low Frequency | Low Energy |
Understanding this relationship opens the door to grasping how waves behave, whether they’re light waves dancing through a prism or sound waves echoing in a concert hall.
The VIBGYOR Spectrum: A Closer Look at Visible Light

When sunlight hits a raindrop or passes through a prism, it splits into a band of colors we call the VIBGYOR spectrum, short for Violet, Indigo, Blue, Green, Yellow, Orange, and Red. This is the visible spectrum wavelength range, stretching from about 380 to 750 nanometers. Each color in this sequence has its own unique wavelength, creating the rainbow’s iconic pattern. The term VIBGYOR, often taught in science classes, captures the order of these colors as they appear when white light is dispersed, making it a handy way to remember the visible light frequency range.
The VIBGYOR rainbow is more than just a pretty sight. It’s a window into how light works. When light travels through a prism, each color bends at a different angle because of its wavelength. Violet light, with the shortest wavelength, bends the most, while red light, with the longest wavelength, bends the least. This dispersion creates the VIBGYOR prism effect, a phenomenon you can recreate at home with a simple glass prism and a flashlight. The visible spectrum range is what our eyes can detect, and it’s just a tiny slice of the broader electromagnetic spectrum, which includes everything from microwaves wavelengths to ultraviolet waves.VIBGYOR Colors and Their Wavelengths

The VIBGYOR Spectrum: Detailed Wavelength & Frequency Analysis
The VIBGYOR sequence is more than just a rainbow; it is a precise physical scale. As we move from Violet to Red, the energy levels shift, and the physical properties of the light change significantly.
1. High-Energy / Short Wavelengths
Violet (380–450 nm): The most energetic color in the visible spectrum. With a frequency of $668–789$ THz, it carries the highest photon energy.
Indigo (450–475 nm): A deep, less distinct hue that bridges the gap between Violet and Blue.
Blue (475–495 nm): Highly scattered by the atmosphere, Blue light is a staple in modern LED technology and laser optics.
2. Mid-Range / High Visibility
Green (495–570 nm): The “Human Peak” Color. Human eyes are biologically most sensitive to Green, which is why it appears the brightest to us.
Yellow (570–590 nm): Positioned in the center of the spectrum, Yellow is highly effective for visibility, often used in street lighting and safety equipment.
3. Low-Energy / Long Wavelengths
Orange (590–620 nm): Known for its warm glow, Orange wavelengths are long enough to be used effectively in traffic signals and atmospheric warning signs.
Red (620–750 nm): The Longest Wavelength. Red has the lowest frequency ($400–484$ THz), allowing it to travel further through fog and dust without scattering which is why “Stop” signs are Red.
| Feature | Violet | Red |
| Wavelength | Shortest ($approx 380$ nm) | Longest ($approx 750$ nm) |
| Frequency | Highest ($approx 789$ THz) | Lowest ($approx 400$ THz) |
| Energy Level | High Energy | Low Energy |
How to Calculate Wavelength

Figuring out wavelength is easier than it sounds, and it’s a skill that comes in handy for students, scientists, and engineers alike. The key formula is λ = c / f, where λ is the wavelength, c is the wave’s speed, and f is its frequency. For light waves, the speed is typically 3×10⁸ meters per second in a vacuum, while for sound waves, it’s about 343 meters per second in air. Let’s walk through a couple of examples to see how this works in real life.
Suppose you’re curious about the wavelength of red light with a frequency of 430 terahertz (4.3×10¹⁴ hertz). Plugging into the formula, you divide the speed of light (3×10⁸ meters per second) by the frequency (4.3×10¹⁴ hertz), giving a wavelength of about 697 nanometers. This matches the visible region wavelength for red light, confirming its place in the VIBGYOR spectrum. For a sound wave, like the 440-hertz note of a tuning fork (the A4 pitch), you divide the speed of sound (343 meters per second) by 440 hertz, resulting in an audio wavelength of roughly 0.78 meters, or 78 centimeters. This wavelength affects how the sound feels in a room, whether it’s crisp or resonant.
You can simplify these calculations using online tools like wavelength calculators or even write a quick Python script to automate the process. For example, a script could take frequency and speed as inputs and spit out the wavelength in seconds, making it a breeze for experiments or homework.
Here’s a table showing the VIBGYOR colors along with their approximate wavelengths and frequencies:| Color | Wavelength Range (nm) | Frequency Range (THz) | Photon Energy (eV) |
| Violet | $380 – 450 | $666 – 789$ | $2.75 – 3.26$ |
| Indigo | $450 – 485$ | $618 – 666$ | $2.56 – 2.75$ |
| Blue | 485 – 500 | 600 – 618 | 2.48 – 2.56 |
| Green | 500 – 565 | 530 – 600 | 2.19 – 2.48 |
| Yellow | 565 – 590 | 508 – 530 | 2.10 – 2.19 |
| Orange | 590 – 625 | 480 – 508 | 1.98 – 2.10 |
| Red | 625 – 750 | 400 – 480 | 1.65 – 1.98 |
VIBGYOR Wavelength Calculator
Enter a wavelength (380 – 750 nm) to see its color and frequency.
 Note: Values are approximate; frequency is calculated using the formula
f = c / λ, where c is the speed of light.
Note: Values are approximate; frequency is calculated using the formula
f = c / λ, where c is the speed of light.The Quantum Relationship: Energy and Wavelength
While the speed equation (λ=c/f) defines the wave’s spatial properties, modern physics requires linking wavelength to the discrete energy carried by each photon—the fundamental particle of light. This relationship is critical in fields like photochemistry and spectroscopy.1. Planck’s Equation (Energy per Photon)
The energy (E) carried by a single photon is inversely proportional to its wavelength (λ). Shorter wavelengths (like Violet) carry higher energy photons than longer wavelengths (like Red).- E= Photon Energy in Joules (J)
- h= Planck’s Constant ( 6.626×10−34 J·s)
- c= Speed of Light ( ≈3.0×108 m/s)
- f= Frequency (Hz) and λ= Wavelength (m)
2. Defining Light Intensity (Irradiance)
Wavelength determines the color, but Intensity determines the brightness or power. Irradiance (I) is the measurable power per unit area delivered by the wave.The Physics of Dispersion: Snell’s Law and the VIBGYOR Effect
The separation of white light into the VIBGYOR spectrum is called Dispersion. This phenomenon is not merely an angle change; it is caused by the dependence of the material’s refractive index (n) on the wavelength ( λ) of the light.1. Quantifying Refraction: Snell’s Law
The angle at which light bends when passing between two media (like air to glass, or air to water) is governed by Snell’s Law. This is the mechanism that determines the path of light through a prism:- n1,n2= Refractive indices of the first and second medium.
- θ1= Angle of incidence.
- θ2= Angle of refraction.
2. The Cause of Dispersion: Refractive Index Dependency
The key to the VIBGYOR spectrum is that the refractive indexnis not a constant, but is slightly different for every color (wavelength). This is quantified by the Cauchy Equation:- Short Wavelengths (Violet): λis smaller, so the term Bλ2is larger, making the refractive index nhigher. A highernmeans the light bends more (θ2is smaller).
- Long Wavelengths (Red): λis larger, making the refractive index nlower. A lower nmeans the light bends less (θ2is larger).
Industry Standards: Wavelength in Telecommunications and Fiber Optics
Outside of visible light, precise control over infrared wavelength is the foundation of modern telecommunications. Fiber optic networks rely on specific, standardized wavelengths to transmit data globally using Wavelength Division Multiplexing (WDM).1. WDM and the ITU Grid
WDM technology allows a single optical fiber to carry multiple, independent communication channels simultaneously. Each channel uses a slightly different, highly specific wavelength. These wavelengths are defined by the ITU-T G.694.1 Grid.The three main transmission windows for minimal signal loss in silica fiber are:- O-Band (Original): Centered around 1310 nm. Used for short-haul transmission.
- C-Band (Conventional): Spanning 1530 nm to 1565 nm. This is the crucial window for long-haul and transatlantic cables.
- L-Band (Long): Spanning 1565 nm to 1625 nm. Used to increase capacity when the C-Band is full.
2. Spectroscopic Standards in Chemistry
In analytical chemistry, VIBGYOR is used for Absorption Spectroscopy. The measured absorption is quantified by the Beer-Lambert Law:- A = Absorbance (unitless)
- ε= Molar absorptivity (M−1 cm−1), a constant specific to the chemical.
- c = Concentration of the absorbing species (M).
- l = Path length (cm).
Exploring the Electromagnetic Spectrum: Ultraviolet and Infrared

The VIBGYOR spectrum is just one part of the vast electromagnetic spectrum, which includes waves we can’t see, like ultraviolet light and infrared radiation. Ultraviolet wavelength, ranging from 10 to 380 nanometers, is shorter than visible light, packing more energy. This makes ultraviolet light wavelength ideal for applications like sterilization, where a UV wavelength of 254 nanometers can kill bacteria in water or on surfaces. Ultraviolet lasers, used in eye surgeries and microfabrication, rely on these short wavelengths for precision, as explained in resources on wave properties in electronics.
Infrared wavelength, on the other hand, stretches from 750 nanometers to 1 millimeter, associated with heat and longer waves. Infrared light radiation powers thermal imaging cameras, letting firefighters see through smoke or wildlife researchers track animals at night. An infrared ray wavelength of 850 nanometers is common in night-vision goggles, while infrared waves wavelength in spectroscopy helps scientists analyze materials. The electromagnetic spectrum includes these and other waves, like microwaves, each with unique roles in technology and science.Wavelength in Action: Real-World Applications

Wavelength isn’t just a concept for text books it’s at work all around us. In optics, the VIBGYOR spectrum is key to spectroscopy, where emission wavelengths reveal the composition of distant stars or chemical samples. You can explore this through light wave diagrams that show how light interacts with matter. In the tech world, ASML EUV lithography uses extreme ultraviolet wavelengths around 13.5 nanometers to etch tiny circuits onto microchips, powering devices like your smartphone, as detailed in ASML’s technology overview.
In acoustics, audio wavelength shapes how sound behaves in spaces like concert halls or recording studios. A low-frequency bass note with a long wavelength might rumble through a room, while a high-pitched note with a shorter wavelength feels sharp, as explored in sound wavelength studies. In electronics, microwaves wavelengths, ranging from 1 millimeter to 1 meter, drive Wi-Fi networks and radar systems, relying on precise wave properties.
Medical fields also harness wavelength. Ultraviolet lasers cut with pinpoint accuracy in surgeries, while infrared radiation wavelength enables non-invasive imaging, like monitoring blood flow. Even in everyday life, wavelength matters think of the orange wavelength in traffic lights or the blue color wavelength in your TV screen. To see wavelength in action, try a simple experiment: shine a flashlight through a prism to split light into the VIBGYOR colors. Notice how each color bends differently, revealing the power of wavelength in shaping light’s behavior.Why Wavelength Matters

Wavelength isn’t just a number it’s a key to understanding the world. The VIBGYOR spectrum shows us how light creates color, from the violet wavelength that dazzles in gemstones to the red colour frequency that glows in sunsets. Beyond beauty, wavelength drives innovation. In industries, infrared wave length powers heat sensors, while ultraviolet light wavelength enables everything from tanning beds to cutting-edge manufacturing. In science, studying light spectrum wavelengths helps astronomers decode the universe’s secrets, as seen in resources like NASA’s spectrum guide. For students, wavelength is a gateway to mastering physics. By experimenting with a light wave diagram or calculating wavelengths, you can see how theory meets reality. For professionals, wavelength shapes fields like telecommunications, where wave properties in electronics guide signal design, or medicine, where precise wavelengths save lives. Even artists use color wavelengths to evoke emotions, from the calming blue color wavelength to the bold orange wavelength.How to Explore Wavelength Yourself
Want to see wavelength in action? Grab a prism and a flashlight to create your own VIBGYOR rainbow. Hold the prism in sunlight or under a white light, and watch the colors fan out. Notice how the violet wavelength bends sharply while the red wavelength stays straighter. You can also experiment with sound: hum a low note and a high note, feeling how the audio wavelength changes the vibration in your throat. For a techy twist, try coding a simple wavelength calculator using Python, or check out online physics modules to simulate wave behavior. If you’re curious about advanced applications, read up on ASML EUV to see how tiny wavelengths power modern electronics. Or explore sound wavelength studies to learn how architects design spaces for perfect acoustics. These hands-on activities make wavelength feel real, connecting abstract science to the world around you.Conclusion
Wavelength is the invisible thread weaving through light, sound, and technology. From the VIBGYOR spectrum’s colorful display to the unseen power of ultraviolet and infrared wavelengths, it shapes how we see, hear, and innovate. By understanding how to calculate wavelength or exploring its role in optics, acoustics, and electronics, you can unlock a deeper appreciation of the universe. Whether you’re marveling at a rainbow, tuning a guitar, or reading about wavelength examples, wavelength is everywhere. So grab a prism, run a calculation, or dive into the light spectrum range the world of wavelength is yours to explore.FAQ : Wavelength and VIBGYOR
Understanding the visible spectrum is fundamental to physics, optics, and telecommunications. Below are the most frequent queries regarding how light and sound waves interact with our world.What does VIBGYOR mean?
It’s a shorthand for the colors of the visible spectrum: Violet, Indigo, Blue, Green, Yellow, Orange, and Red. These are seen in rainbows or when white light passes through a prism.Which color has the highest wavelength?
Red light, with wavelengths up to 750 nm, takes the crown for the longest wavelength in the VIBGYOR spectrum.What’s the visible light wavelength range?
The visible spectrum spans roughly 380 to 750 nanometers, covering all VIBGYOR colors detectable by the human eye.How do you calculate wavelength?
You use the fundamental wave equation:What’s the wavelength of blue color?
Blue light falls between 475and 495 nanometers, giving it that crisp, vibrant look used in modern display technologies.Which color has the maximum frequency?
Violet light, with frequencies of 668to 789 THz, has the highest frequency in the visible spectrum, making it the most energetic.What’s the wavelength of yellow colour?
Yellow light ranges from 570to 590 nanometers, creating its bright, cheerful glow.What’s the frequency of red colour?
Red light’s frequency is between 400and 484 terahertz, which is the lowest frequency in the VIBGYOR sequence.Which colour has the shortest wavelength?
Violet, with wavelengths as low as 380 nm, is the shortest in the visible spectrum.What’s the wavelength of orange colour?
Orange light spans 590 to 620 nanometers, perfect for warm, inviting hues.How does a prism create VIBGYOR?
A prism bends light based on wavelength (refraction). Shorter wavelengths like violet bend more, while longer ones like red bend less, producing the separated rainbow spectrum.What’s the difference between visible rays and other waves?
Visible rays are the only part of the electromagnetic spectrum our eyes can detect ( 380– 750 nm). Other waves, like Ultraviolet (UV) or Infrared (IR), fall outside this human detection range.What’s the wavelength of green colour?
Green light, from 495to 570 nanometers, is the most visible and sensitive to the human eye.Pro Tip: Wavelength significantly impacts technology—from microwave communication to UV sterilization. These queries, inspired by “People Also Ask” trends, show how deeply wavelength connects to science and daily life.