Table of Contents
- 1. What is the Periodic Table of Elements? Why Does It Matter?
- 2. The Evolution: From Ancient Ideas to Mendeleev
- 3. Understanding Groups, Periods, and Blocks
- 4. Quantum Foundation: Atomic Energy & States
- ↳ Atomic Energy Levels (Bohr Model)
- ↳ The Four Quantum Numbers
- 5. Full Periodic Table (All 118 Elements)
- 6. Nuclear Physics: Mass Defect & Decay
- ↳ Mass Defect and Binding Energy (Δm)
- ↳ Radioactive Decay and Half-Life (t1/2)
- 7. Periodic Trends: Atomic Size & Electronegativity
- 8. Full List of Elements (First 20 and Beyond)
- 9. Real-World Uses & Mind-Blowing Facts
- 10. Advanced Apps: Electrochemistry & Spectroscopy
- 11. Frequently Asked Questions (FAQ)
Did you know the periodic table organizes over 118 elements that make up everything from your smartphone to the stars? This single chart is the ultimate cheat sheet of chemistry and today you’re going to master it.
What is the Periodic Table? Why Does It Matter?
The periodic table of elements is a systematic arrangement of all known chemical elements ordered by increasing atomic number. When elements are arranged this way, they display recurring (periodic) patterns in their chemical and physical properties. Think of it as the “DNA of matter.” Just as the alphabet organizes 26 letters into infinite words, the periodic table organizes 118 elements into every substance in the universe.
Whether you’re a student preparing for exams, a teacher looking for the perfect resource, or just curious about the building blocks of reality, understanding the periodic table unlocks the secrets of chemistry. From predicting how elements react to designing new materials for electric cars, the periodic table is used daily by scientists, engineers, and doctors.
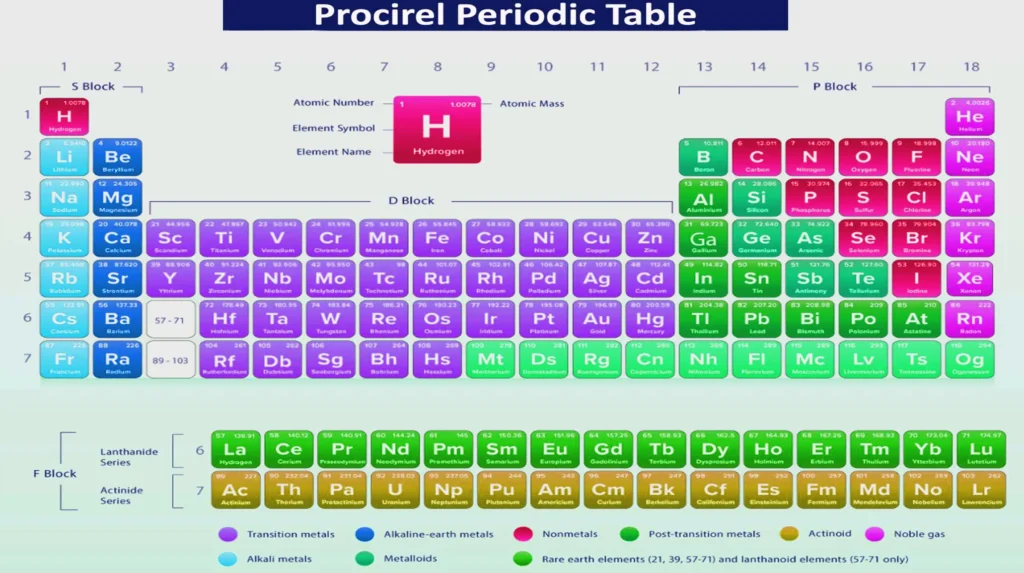
Modern Periodic Table: Color-Coded for Easy Learning
The Evolution of the Periodic Table From Ancient Ideas to Mendeleev’s Breakthrough
The journey to the modern periodic table took over a century of brilliant discoveries.
Early Attempts
- 1789 – Antoine Lavoisier grouped elements into gases, metals, non-metals, and earths.
- 1829 – Johann Döbereiner discovered “triads” (e.g., lithium, sodium, potassium) with similar properties.
- 1864 – John Newlands proposed the “Law of Octaves” — every eighth element had similar properties (like musical notes).
Mendeleev’s Genius (1869)
In 1869, Russian chemist Dmitri Mendeleev created the first widely accepted periodic table. What made his version revolutionary?
- He arranged elements by increasing atomic mass (later corrected to atomic number).
- He left gaps for undiscovered elements and even predicted their properties!
- Example: He predicted “eka-silicon” — discovered in 1886 as germanium, matching his predictions almost exactly.
The Modern Breakthrough
In 1913, Henry Moseley discovered that atomic number (number of protons) — not atomic mass — is the true basis for ordering elements. This fixed inconsistencies in Mendeleev’s table and gave us the modern periodic table we use today.
Timeline: How the Periodic Table Was Born
Understanding Groups, Periods, and Blocks in the Periodic Table
The periodic table isn’t random it’s organized into rows and columns with deep meaning.
Periods (Horizontal Rows)
There are 7 periods. As you move left to right across a period:
- Atomic number increases by 1
- Electrons are added to the same energy level
- Elements become less metallic and more non-metallic
Groups (Vertical Columns)
There are 18 groups. Elements in the same group have:
- Same number of valence electrons
- Similar chemical properties
- Predictable reactivity patterns
Key Groups You Must Know
- Group 1: Alkali metals (Li, Na, K) — extremely reactive with water
- Group 2: Alkaline earth metals (Mg, Ca) — reactive but less than Group 1
- Group 17: Halogens (F, Cl, Br) — highly reactive non-metals
- Group 18: Noble gases (He, Ne, Ar) — very stable, almost inert
Blocks: s, p, d, f
The table is divided into blocks based on the orbital being filled:e
- s-block: Groups 1–2
- p-block: Groups 13–18
- d-block: Transition metals
- f-block: Lanthanides and actinides (usually shown separately)
Groups and Periods: Key to Element Families
Quantum Foundation: Atomic Energy and Electron State
The periodic table’s structure its periods, groups, and blocks is entirely defined by quantum mechanics. To move beyond descriptive chemistry, an engineer must quantify the energy and location of electrons.
1. Atomic Energy Levels (Bohr Model Extension)
The energy required to excite or remove an electron is the basis for ionization energy, one of the key periodic trends. For a hydrogen-like atom, the electron energy level
En
is defined by its principal quantum number (
n):
E
n=
−Z2
REn2
Where:
- Z= Atomic Number (number of protons)
- n= Principal Quantum Number (1, 2, 3…)
- RE= Rydberg Energy Constant(2.18×10−18 J)
Insight: This formula quantifies the stability of the inner shells (low
n) versus the outer valence shells (high
n).
2. The Four Quantum Numbers
These four numbers mathematically describe the unique state of any single electron in an atom, precisely explaining the s, p, d, and f block structure:
- Principal Quantum Number (
n):Defines the energy level (shell) and size. Corresponds to the Period number. (n=1,2,3…) - Angular Momentum (
l):Defines the shape of the orbital (subshell). Corresponds to the Block. (l=0⇒s-orbital,l=1⇒p-orbital, etc.) - Magnetic Quantum Number (
ml):Defines the orientation of the orbital in space. (ml=−l…+l) - Spin Quantum Number (
ms):Defines the electron’s spin (±1/2)
.
Understanding these numbers is essential for predicting complex chemical bonds and spectroscopic properties.
Full Periodic Table (All 118 Elements)
| 1 | H – Hydrogen | 2 | He – Helium | 3 | Li – Lithium | 4 | Be – Beryllium | 5 | B – Boron |
| 6 | C – Carbon | 7 | N – Nitrogen | 8 | O – Oxygen | 9 | F – Fluorine | 10 | Ne – Neon |
| 11 | Na – Sodium | 12 | Mg – Magnesium | 13 | Al – Aluminum | 14 | Si – Silicon | 15 | P – Phosphorus |
| 16 | S – Sulfur | 17 | Cl – Chlorine | 18 | Ar – Argon | 19 | K – Potassium | 20 | Ca – Calcium |
| 21 | Sc – Scandium | 22 | Ti – Titanium | 23 | V – Vanadium | 24 | Cr – Chromium | 25 | Mn – Manganese |
| 26 | Fe – Iron | 27 | Co – Cobalt | 28 | Ni – Nickel | 29 | Cu – Copper | 30 | Zn – Zinc |
| 31 | Ga – Gallium | 32 | Ge – Germanium | 33 | As – Arsenic | 34 | Se – Selenium | 35 | Br – Bromine |
| 36 | Kr – Krypton | 37 | Rb – Rubidium | 38 | Sr – Strontium | 39 | Y – Yttrium | 40 | Zr – Zirconium |
| 41 | Nb – Niobium | 42 | Mo – Molybdenum | 43 | Tc – Technetium | 44 | Ru – Ruthenium | 45 | Rh – Rhodium |
| 46 | Pd – Palladium | 47 | Ag – Silver | 48 | Cd – Cadmium | 49 | In – Indium | 50 | Sn – Tin |
| 51 | Sb – Antimony | 52 | Te – Tellurium | 53 | I – Iodine | 54 | Xe – Xenon | 55 | Cs – Cesium |
| 56 | Ba – Barium | 57 | La – Lanthanum | 58 | Ce – Cerium | 59 | Pr – Praseodymium | 60 | Nd – Neodymium |
| 61 | Pm – Promethium | 62 | Sm – Samarium | 63 | Eu – Europium | 64 | Gd – Gadolinium | 65 | Tb – Terbium |
| 66 | Dy – Dysprosium | 67 | Ho – Holmium | 68 | Er – Erbium | 69 | Tm – Thulium | 70 | Yb – Ytterbium |
| 71 | Lu – Lutetium | 72 | Hf – Hafnium | 73 | Ta – Tantalum | 74 | W – Tungsten | 75 | Re – Rhenium |
| 76 | Os – Osmium | 77 | Ir – Iridium | 78 | Pt – Platinum | 79 | Au – Gold | 80 | Hg – Mercury |
| 81 | Tl – Thallium | 82 | Pb – Lead | 83 | Bi – Bismuth | 84 | Po – Polonium | 85 | At – Astatine |
| 86 | Rn – Radon | 87 | Fr – Francium | 88 | Ra – Radium | 89 | Ac – Actinium | 90 | Th – Thorium |
| 91 | Pa – Protactinium | 92 | U – Uranium | 93 | Np – Neptunium | 94 | Pu – Plutonium | 95 | Am – Americium |
| 96 | Cm – Curium | 97 | Bk – Berkelium | 98 | Cf – Californium | 99 | Es – Einsteinium | 100 | Fm – Fermium |
| 101 | Md – Mendelevium | 102 | No – Nobelium | 103 | Lr – Lawrencium | 104 | Rf – Rutherfordium | 105 | Db – Dubnium |
| 106 | Sg – Seaborgium | 107 | Bh – Bohrium | 108 | Hs – Hassium | 109 | Mt – Meitnerium | 110 | Ds – Darmstadtium |
| 111 | Rg – Roentgenium | 112 | Cn – Copernicium | 113 | Nh – Nihonium | 114 | Fl – Flerovium | 115 | Mc – Moscovium |
| 116 | Lv – Livermorium | 117 | Ts – Tennessine | 118 | Og – Oganesson |
Nuclear Physics: Mass Defect and Radioactive Decay
The very stability and existence of heavier elements are governed by forces within the nucleus. Nuclear physics provides the formulas for quantifying the energy stored within an atom and the rate at which unstable isotopes decay.
1. Mass Defect and Binding Energy (Δm)
The mass of an atom’s nucleus is always less than the sum of the masses of its individual protons and neutrons. This “missing mass” (Δm) is converted into the energy required to hold the nucleus together (binding energy), calculated via Einstein’s mass–energy equivalence.
Binding energy formula
E= Δm c2
Where:
- Δm = mass defect (difference between sum of constituent nucleon masses and actual nuclear mass)
- c = speed of light in vacuum
Insight: The binding energy per nucleon peaks around Iron (
Fe-56). Elements lighter than
Fe-56release energy through fusion, and elements heavier than
Fe-56release energy through fission.
2. Radioactive Decay and Half-Life ( t1/2)
The decay of unstable radioactive isotopes follows first‑order kinetics. The half‑life t1/2 is the time required for half the original sample N0 to decay. The decay law and the half‑life relation are shown below.
Radioactive decay law
N(t)= N_0 e −λt Half‑life relation : html t_1/2= ln(2) λ
Where:
- N(t) is the amount remaining at time t
- N0 is the initial amount at t=0
- λ is the decay constant (probability per unit time of decay)
- t1/2 is the half‑lifeNotes and practical implications:
- The decay constant λ and half‑life t1/2 are inversely related; a large λ means a short half‑life.
- Binding energy per nucleon and mass defect explain why fusion powers stars (light elements) and why fission of very heavy nuclei releases energy.
- For calculations involving binding energy per nucleon, convert mass defect (in atomic mass units or kilograms) to energy using the mass–energy relation above and, if needed, divide by the number of nucleons to get per‑nucleon values.
Understanding mass defect, binding energy, and radioactive decay kinetics provides the quantitative foundation for nuclear stability, energy release mechanisms (fusion and fission), and the time scales over which radioactive processes occur.
Periodic Table Trends: Atomic Size, Electronegativity, Ionization Energy Explained
Trends are the “superpowers” of the periodic table — they let you predict properties without memorizing everything.
Major Trends Summary
| Trend | Across a Period (→) | Down a Group (↓) |
|---|---|---|
| Atomic Radius | Decreases | Increases |
| Electronegativity | Increases | Decreases |
| Ionization Energy | Increases | Decreases |
| Metallic Character | Decreases | Increases |
Why These Trends Happen
- Across a period: More protons pull electrons closer → smaller size
- Down a group: Extra electron shells → larger size
Real-World Examples
- Lithium (small battery) vs Francium (huge and super reactive)
- Fluorine (most electronegative) steals electrons → powerful cleaning agents
- Cesium used in atomic clocks because of predictable low ionization energy
Visualizing Periodic Trends for Quick Mastery
Full List of Periodic Table Elements – First 20 and Beyond
First 20 Elements (Most Important for Students)
- Hydrogen (H) – Lightest element, fuel of the future
- Helium (He) – Used in balloons and MRI machines
- Lithium (Li) – Powers your phone battery
- Beryllium (Be) – Lightweight metal for aerospace
- Boron (B) – Makes glass heat-resistant
- Carbon (C) – Basis of all life
- Nitrogen (N) – 78% of the air you breathe
- Oxygen (O) – Essential for respiration
- Fluorine (F) – In your toothpaste
- Neon (Ne) – Glows in signs
- Sodium (Na) – Table salt (NaCl)
- Magnesium (Mg) – In chlorophyll and fireworks
- Aluminum (Al) – Lightweight metal for cans
- Silicon (Si) – Basis of computer chips
- Phosphorus (P) – In DNA and matches
- Sulfur (S) – Smell of rotten eggs
- Chlorine (Cl) – Disinfects swimming pools
- Argon (Ar) – Most common noble gas in air
- Potassium (K) – Essential for nerve function
- Calcium (Ca) – Builds strong bones
Free Printable Periodic Table – Perfect for Classrooms
Real-World Uses of the Periodic Table and Mind-Blowing Facts
- Gold (Au) and silver (Ag) are in the same group as copper — all excellent conductors
- Carbon forms diamonds and graphene — same element, wildly different properties
- Oganesson (Og) is the only element named after a living scientist (Yuri Oganessian)
- There are more atoms in a grain of sand than stars in the observable universe
- Technetium (Tc) was the first artificially produced element

Advanced Applications: Electrochemistry and Spectroscopy
The chemical properties detailed in the periodic table are quantified using formulas that govern electrochemical potential (batteries) and light emission (spectroscopy).
1. The Nernst Equation (Battery Voltage)
Engineers use the Nernst Equation to calculate the voltage
Ecell of any electrochemical cell (battery, fuel cell) under non-standard conditions, taking into account temperature (T) and the concentrations of reactants (Q): E cell= E cell ∘ − R T z F ln ( Q )
Where:
- Ecell∘= Standard Cell Potential (V)
- R= Universal Gas Constant
- T= Temperature (K)
- z= Moles of electrons transferred
- F= Faraday Constant
Industry Standard: This is the quantitative backbone of battery design and corrosion science, linking the thermodynamic potential of elements (like Lithium, Zinc) to real-world performance.
2. Rydberg Formula (Element Fingerprints)
Each element emits and absorbs light at specific, unique wavelengths, which act as its atomic “fingerprint”. The Rydberg formula quantifies this spectral signature:
1 λ= Z2 R ∞ ( 1 n12 − 1 n22 ) Where λis the wavelength, Zis the atomic number, and R∞is the Rydberg constant. This allows the precise identification of elements in remote stars or chemical samples.
Elements in Everyday Life: From Your Phone to the Stars
Frequently Asked Questions About the Periodic Table
Q1: What are the first 20 elements of periodic table in order?
- H – Hydrogen
- He – Helium
- Li – Lithium
- Be – Beryllium
- B – Boron
- C – Carbon
- N – Nitrogen
- O – Oxygen
- F – Fluorine
- Ne – Neon
- Na – Sodium
- Mg – Magnesium
- Al – Aluminium
- Si – Silicon
- P – Phosphorus
- S – Sulphur
- Cl – Chlorine
- Ar – Argon
- K – Potassium
- Ca – Calcium
Q:2 How many elements are there in periodic table?
118 Elements are present in the Periodic Table. As per the periodic law, the properties of Elements are periodic functions of their atomic numbers.
Q:3 Who created the periodic table and why?
The structure for the contemporary periodic table was developed in 1869 by Russian chemist Dmitri Mendeleev, who left spaces for elements that had not yet been discovered.
Q:4 What is a group in a periodic table?
A group is a column of elements in the periodic table of the chemical elements. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms.
Q:5 What is modern periodic table explain?
The modern or long form of the periodic table is based on the modern periodic law. The table is the arrangement of elements in increasing order of their atomic numbers. The modern periodic table is the present form of the periodic table. And it consists of 18 vertical columns and 7 horizontal rows.
Q:6 Who found the first element of Periodic table?
In 1669, phosphorus was the first element to be chemically discovered by Hennig Brandt .
Q:7 How many metals and non-metals are there in 118 elements?
There are 118 different elements in the current periodic table. There are 18 non-metals. There are 7 metalloids and 93 different types of metal.
Q:8 What are the 4 blocks on the periodic table?
The valence electron orbitals of the elements in the periodic table are used to group them into blocks. The four blocks are s-block, p-block, d-block, and f-block
Q:9 What are the 4 trends of the periodic table?
There are four main periodic trends, electronegativity, atomic size, ionization energy, and electron affinity.
Q:10 How was the atomic number discovered?
Henry Moseley made the discovery of the atomic number in 1913 while analysing X-ray spectra. He discovered that when we increase the atomic number by one, certain lines in the x-ray spectra of atoms move by the same amount each time.


excellent post, very informative. I wonder why the other specialists of this sector do not notice this. You should continue your writing. I am confident, you have a huge readers’ base already!